The family of a toddler with a life-limiting muscle disorder have backed a campaign for other people to receive a new drug which saved their boy’s life.
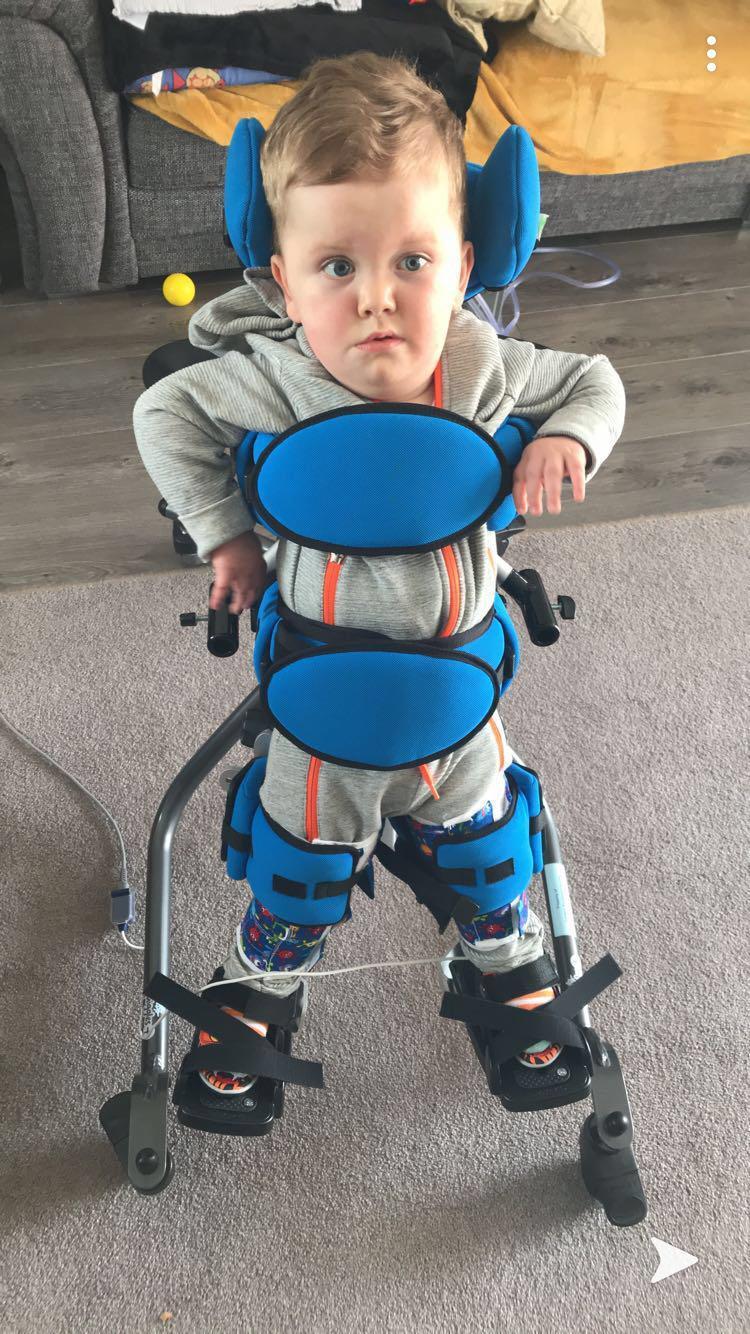
Kylan-J Heslop, of Thornhill, has an extremely rare condition known as Spinal Muscular Atrophy (SMA) type one but has made huge strides since he started receiving new treatment when he was six-months-old.
Now nearly two, the youngster’s quality of life has improved significantly since the beginning of the trial of Spinraza, which is also known as nusinersen.
His improvement has inspired mum Amy Thomas to join an appeal by the charity TreatSMA against criteria suggested by the National Institute for Health and Care Excellence (NICE) to the NHS for who should receive the drug.
Amy, 32, said: “Kylan gets the drug regardless; he was in the trial which proved successful and it was approved for all types of SMA in May.
“Now NICE have revealed some stipulations that people would have to meet to qualify for the drug.
“Some people did meet the criteria but it is a progressive disease and in the time they have waited for this to come in, things have got worse and they now wouldn’t get it.
“It made a life or death difference for Kylan; he would have died without it.
“We were told we’d lose him and a week later after having the drug he’d made massive improvements.”
The draft guidance put forward by NICE to the NHS includes criteria for patients to meet such as being able to take at least five steps independently and a requirement for patients not to be dependent on a certain amount of ventilation.
A spokesperson for NICE confirmed an appeal had been received and said they understood the eligibility was “disappointing for patients who may not meet the criteria.”
They said: “We will now, in line with our process, review the appeal points raised to decide whether it can be considered by an appeal panel.
“This has been a challenging appraisal and all parties – NHS England, NICE and Biogen – worked together extensively to reach an arrangement that allows access to nusinersen for most people with SMA type one, two, three and pre-symptomatic patients.”





Comments: Our rules
We want our comments to be a lively and valuable part of our community - a place where readers can debate and engage with the most important local issues. The ability to comment on our stories is a privilege, not a right, however, and that privilege may be withdrawn if it is abused or misused.
Please report any comments that break our rules.
Read the rules here